The Big Bang …and All That Follows!
By Damon Kowarsky
In 2019, to celebrate International Year of the Periodic Table and the 150th anniversary of Dmitri Mendeleev’s 1869 discovery, Melbourne artists Damon Kowarsky and Hyunju Kim were commissioned to design 51 images describing the birth of the universe through the creation of the elements of the Periodic Table by Soula Bennett, Director of Quantum Victoria, a specialist Science and Mathematics Centre established by the Victorian Department of Education and Training.
These 51 images were installed on hexagons in the gallery at Quantum Victoria and launched in July 2019. In 2020, as the first COVID restrictions took place, Damon Kowarsky was further commissioned to produce a series of essays exploring the images and their relations to science, history, culture, technology, and politics.
The first and last of the 51 images depict the Big Bang and Mendelevium and begin and conclude the broader narrative of the birth of the universe through to the charting of the elements on the Periodic Table.
 There are many problems in depicting the beginning of the universe. After all, it happened 13.8 billion years ago, and no one was there to watch. Indeed, what is known about the Big Bang has been extrapolated from observations of the current universe – its expansion, levels of background heat and radiation, the prevalence of the primordial isotopes of hydrogen, helium, and lithium.
There are many problems in depicting the beginning of the universe. After all, it happened 13.8 billion years ago, and no one was there to watch. Indeed, what is known about the Big Bang has been extrapolated from observations of the current universe – its expansion, levels of background heat and radiation, the prevalence of the primordial isotopes of hydrogen, helium, and lithium.
There is also the matter of conception. Although the word atom means indivisible, we have long understood them to be formed of smaller parts – protons, neutrons, and electrons. Now we realise these subatomic particles are only three kinds of baryons [subatomic particles that have a mass equal to or greater than that of a proton] and are themselves divisible into six types of quarks [subatomic particles that make up matter]. And all this, infinitely dense and infinitely hot, was condensed along with space and time into a single, infinitely small, point.
Most depictions of the Big Bang show an explosion, the moment when the universe expanded out from its initial singularity, but there’s a problem here too. An explosion is best seen from somewhere else, and as the singularity contained not only all matter and energy but all space and time, there was no “outside” to witness it from. Literally, there was nowhere to rest that hypothetical camera.
As a visual artist, I have attempted to represent all these conceptually difficult concepts in a single image. Also contained in this image is the elegant curve of the Fibonacci spiral, a spiral that increases in size by the numbers of the Fibonacci sequence [1, 1, 2, 3, 5, 8, 13, 21, etc.]. Along its length are significant events in the timeline of the universe. On the right, ten or so billion years ago, is the formation of the disk of the Milky Way. To the left, 4.6 billion years ago, is our Sun. Our species enters the scene just as the curve intersects the image’s hexagonal border.
All the existing elements were formed following the Big Bang. Some in the early stages of the universe, others as stars burned fuel or exploded. Still others were discovered or created in the developments of the Nuclear Age. The 51 elements depicted at Quantum Victoria are important to us – not least because we wouldn’t be here without them – because we use them, actually consume them, in some way or another. But for this essay, let’s jump to the last element depicted – Mendelevium.
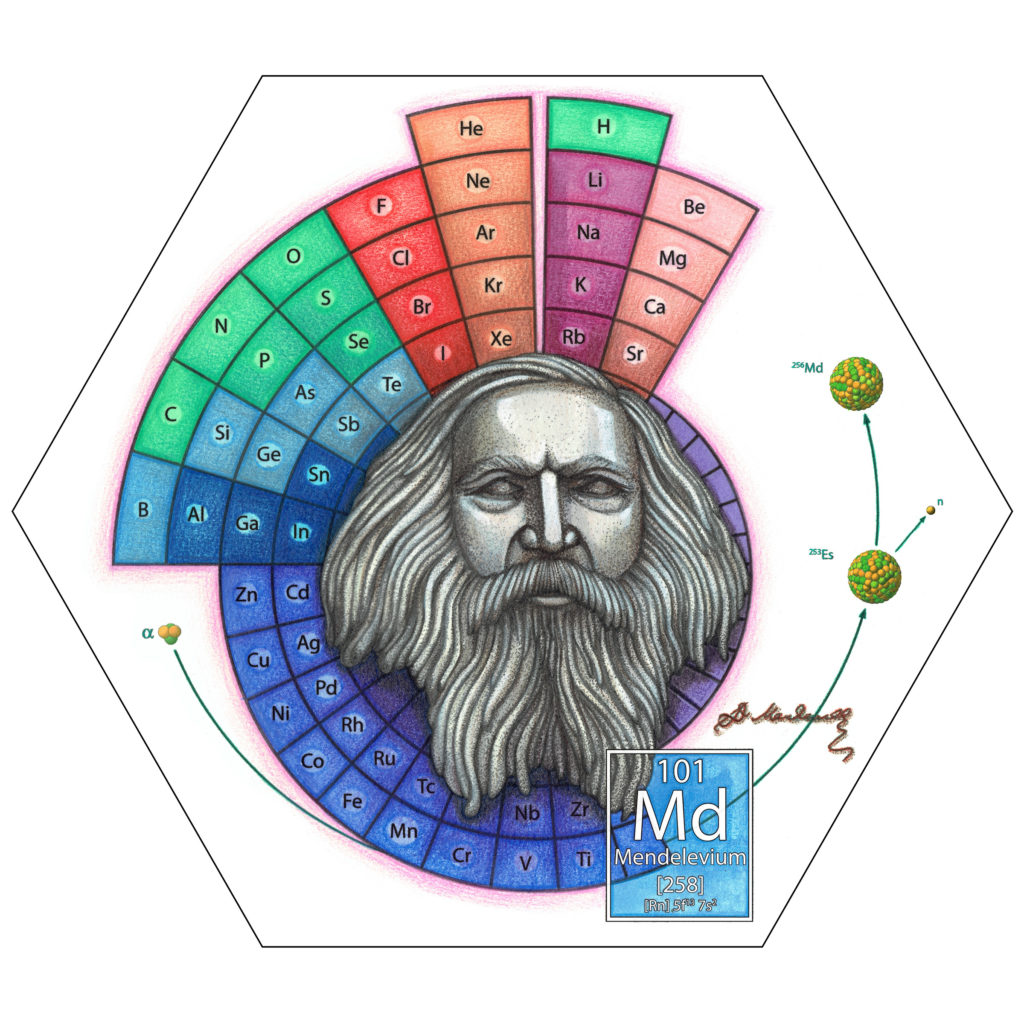
In 1848 Dmitri Mendeleev left his birthplace in Tobolsk in western Siberia and travelled with his mother 2,300 km to Moscow. They hoped to enrol him at Moscow University and improve the family situation. Mendeleev’s schoolteacher father had gone blind, and the family business had burnt down.
It was not to be. Maria Dmitrievna Mendeleeva and her son travelled another 700 km to Saint Petersburg where he was accepted into the teachers’ college at the Imperial University. After graduating Mendeleev taught in Simferopol on the Black Sea and studied liquids and spectroscopy in the German city of Heidelberg. In 1864 Mendeleev became a professor at Saint Petersburg Technological Institute and State University.
The question of how to arrange the elements had been around since at least 1789 when French chemist Antoine Lavoisier sought to classify them into metals and non-metals. In 1817 the German physicist Johann Wolfgang Döbereiner grouped the elements into triads including lithium, sodium, potassium, and calcium, strontium, barium.
By 1864 the English chemist John Newlands had grouped the elements according to intervals of eight. He was ridiculed by the Chemical Society of London for doing so.
In 1869 Mendeleev arranged the 63 known elements according to their atomic weights. In doing so he saw patterns. What we now call halogens were always followed by alkali metals, then alkaline earth metals. These patterns held up even after William Ramsay discovered noble gases in the 1890s.
As well as arranging the elements Mendeleev made two important breakthroughs. The first was to reverse the order of iodine and tellurium. While tellurium is heavier than iodine its properties are closer to selenium, and iodine’s to bromine. It was not until 1932, and James Chadwick’s discovery of the neutron, that the difference between atomic mass and atomic number was resolved.
The second was to leave space for elements unknown. Many of these Mendeleev predicted. Eka-aluminium became gallium, eka-silicon germanium. Although Mendeleev never received a Nobel Prize, in 1955 he became part of a much rarer group. The 101st element was named for him, making Mendeleev one of only 16 people to ever be honoured in this way.
An Elemental Journey Through the Periodic Table is on permanent public display at Quantum Victoria at Charles La Trobe College, Macleod West.








