Lighting the Way: The Australian Synchrotron
By Dale Christensen
Australian Nuclear Science & Technology Organisation (ANSTO)
From 1957 to 1984, Melburnians flocked in their thousands to the corner of Wellington and Blackburn Roads in Clayton. Here they peered up at massive steel structures, illuminated by powerful and expensive light sources – the Clayton Metro Twin drive-in theatre was as much a cultural icon as it was a technological and engineering marvel.
After a less glamorous interlude as a university car park, the site re-opened in 2007 as home to the Australian Synchrotron. Gone are the floodlight towers and enormous concave screens; in their place stands a particle accelerator the size of a footy field. The twin projectors have been replaced with a state-of-the-art light source a million times brighter than the Sun. The unassuming frontage along Blackburn Road masks a hidden gem of Australia’s research infrastructure.

What is the Australian Synchrotron?
At its simplest, a synchrotron is a light source – illuminating samples of scientific interest with x-ray and infrared radiation. This ‘synchrotron radiation’ is produced when electrons are accelerated close to the speed of light and forced by strong magnetic fields to travel in a circular orbit. Light produced in this way is extremely bright, focused, and collimated (meaning that it doesn’t spread out much as it travels). This allows scientists from around Australia to analyse their samples with greater resolution and on smaller timescales than is available through more conventional techniques.
The Australian Synchrotron is managed by the Australian Nuclear Science & Technology Organisation (ANSTO), and is the only synchrotron in the country. In fact, it is one of only two in the southern hemisphere, making it a melting pot of scientific inquiry, where some of the most challenging research questions are brought under one roof.
The Synchrotron welcomes about 5,000 scientists a year, investigating questions like: What geochemical factors help explain the development of the Kimberley’s famous zebra rock formations?1 Can we rapidly detect coronavirus markers in a saliva swab?2 How can we modify cacao bean processing to reduce the amount of cadmium in chocolate?3
When visitors tour the facility, Synchrotron Director Prof. Michael James likes to play a game: “Pick a topic, any topic”, he says, “and I will tell you how we make a difference to that, by research carried out at the Australian Synchrotron.” In the spirit of that game, and in keeping with the theme of this issue of Science Victoria, allow me to give you a taste of some of the fascinating research being performed at the Synchrotron relating to Victorian fauna.
Researching Victoria’s fauna at the Synchrotron
The plight of the critically endangered orange-bellied parrot has received a lot of publicity over the last decade, especially regarding the threat posed by wind turbines in its migratory path.4 Unfortunately, this is not the only peril facing the iconic avian: psittacine beak and feather disease (PBFD) has devastated populations of this and other endangered parrots. PBFD results in beak and feather abnormalities, and is often fatal. It is caused by the highly infectious psittacine circovirus (PCV), which has only two genes: one for self-replication, and another to form sixty copies of a ‘capsid’ protein, which then self-assemble into a shell to contain the virus.
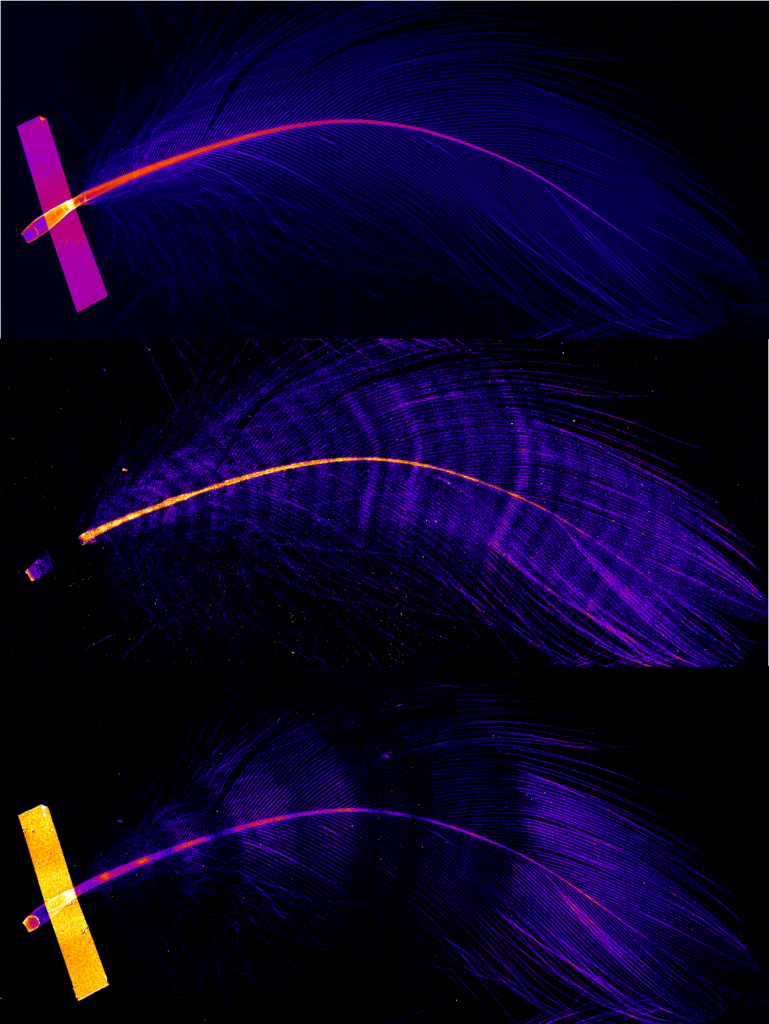
Top: density of structures within the feather (lighter colour = more dense).
Middle: zinc distribution. The number of zinc bands found in these feathers (30-32) corresponds to the number of days it takes to grow a feather, suggesting one band every 24 hrs.
Bottom: bromine density bands are irregular, and might be caused by dietary or environmental changes as the birds migrate.
Image: Nick Howell, ANSTO biologist.
Scientists from Charles Sturt University, Monash University, Spain’s National Microbiology Centre, and the Autonomous University of Madrid used the Micro Crystallography (MX2) beamline at the Synchrotron to analyse this capsid protein.5 The interaction between synchrotron x-rays and an assembly of capsid proteins produced diffraction patterns – complex forms that can be used to calculate the precise molecular structure of the protein in question. This is an important step towards an eventual PBFD vaccine and treatment: if we know the structure of the capsid protein, we can pharmaceutically target key parts of it to disrupt viral replication and abort infection, or can design a vaccine that protects against infection in the first place.
Sticking with our migratory birds, the X-ray Fluorescence Microscopy (XFM) beamline has been used to analyse the feathers of three seabird species: the Flesh-footed, Short-tailed, and Streaked shearwaters.6 Different chemical elements give off different light signatures, which the beamline uses to produce nanometre-scale maps of where these elements are distributed within a sample.
Scientists from James Cook University and the University of Tasmania were able to identify regular banding patterns of zinc within the shearwater feathers (Figure 1). These patterns were independent of mass, density, and colour changes within the feather, suggesting that this regular variation in zinc may be a part of the feather development process – somewhat like the rings in a tree trunk. If this is accurate, scientists may be able to use abnormalities in this pattern to detect stressors in a bird’s recent past, such as dietary changes, or exposure to environmental contaminants. This analysis could also be completely non-invasive, since moulting is a natural process in birds.
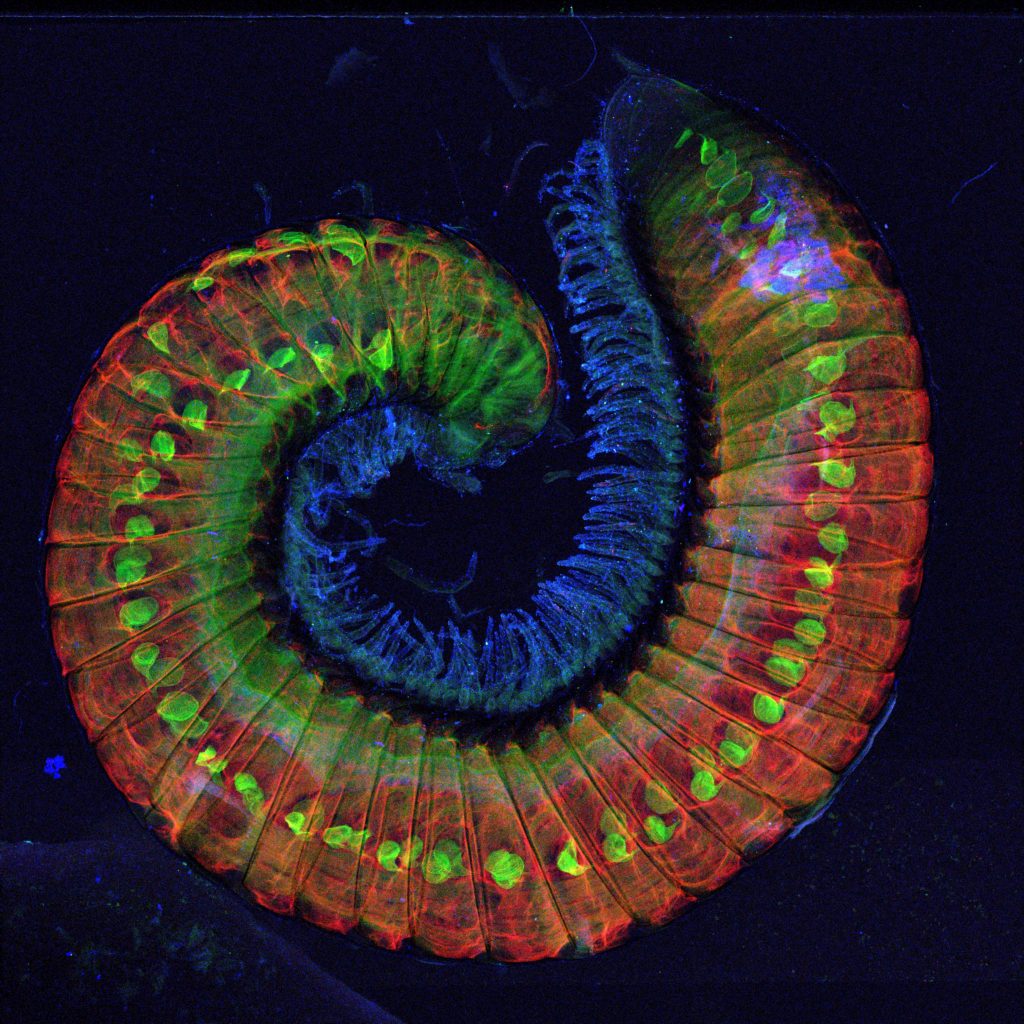
The XFM team has analysed more than just birds: other highlights in Australian Animalia include the invasive millipede Ommatoiulus moreleti (Figure 2), and even diprotodon (ancient wombat-like megafauna) fossils, though this work is yet to be published. The Imaging and Medical beamline (IMBL) at the Synchrotron has also been used to study a variety of Australian animals, including possums and wallabies.
A bright future
With all this exciting scientific output, the Synchrotron’s science team are not resting on their laurels but looking forward to further illuminating the research questions of tomorrow.
Dr Mitzi Klein, a staff veterinarian at the Synchrotron, is hoping to use the IMBL beamline to produce a ‘digital zoo’ – a thorough compendium of high-resolution scans of native fauna. “There are many, many species that have never been imaged at all,” she said. “The value of a virtual dissection cannot be underestimated. These species are rare, and of value intact to museums. To have a collection of synchrotron scans would enable comparisons between species (that are often lumped together as being similar), and also within species. That information would be invaluable to all sorts of specialists – zoologists, ecologists, palaeontologists, and so on.”
When you drive down Blackburn Rd in 2024, you won’t see the drive-in theatre light towers, or the nostalgic “SHOWING TONIGHT:” sign. Instead, you’ll pass the Australian Synchrotron: a crucial hub in the nation’s research network. Without it, many Australian academics would be limited to data obtained from benchtop instruments, or be forced to make expensive international trips to synchrotron facilities elsewhere. We’re building more beamlines, adding greater capabilities, and looking forward to answering Australia’s most critical scientific questions for years to come.
—
Dr Dale Christensen is an analytical chemist by training, gaining a PhD from Monash University for his research in infrared biospectroscopy of dengue-carrying mosquitoes. He now works for ANSTO as an Accelerator Operator in the control room of the Australian Synchrotron.
References:
- Coward, A. J., et al. (2023). Mineralogy and geochemistry of pattern formation in zebra rock from the East Kimberley, Australia. Chemical Geology, 622, 121336–121336. doi.org/10.1016/j.chemgeo.2023.121336
- Wood, B. R., et al. (2021). Infrared Based Saliva Screening Test for COVID‐19. Angewandte Chemie International Edition, 60(31), 17102–17107. doi.org/10.1002/anie.202104453
- Vanderschueren, R., et al. (2020). The impact of fermentation on the distribution of cadmium in cacao beans. Food Research International, 127, 108743. doi.org/10.1016/j.foodres.2019.108743
- Reid, K., et al. (2023). An ecological risk assessment for the impacts of offshore wind farms on birds in Australia. Austral Ecology. doi.org/10.1111/aec.13278
- Sarker, S., et al. (2016). Structural insights into the assembly and regulation of distinct viral capsid complexes. Nature Communications, 7(1), 13014. doi.org/10.1038/ncomms13014
- Howell, N. R., et al. (2017). The Topobiology of Chemical Elements in Seabird Feathers. Scientific Reports, 7(1). doi.org/10.1038/s41598-017-01878-y





